
Tuesday, December 15, 2015
Copper Chloride Lab Day 2
On day 2 of the Copper Chloride & Iron Lab the copper had grown a lot over night. It was very interesting to see what was once a thin layer of copper on the nail, had about tripled in size in one day.


Copper Chloride Lab
Stoichiometry Lesson
The lessons we learn about stoichiometry aren't hard to understand. For me it's just applying them that get's tricky. Everything has been pretty easy as it just follows a few basic steps, and as long as you memorize which steps to take and when, it can be easily done. Other than that, all you need is knowing conversion skills which we learned in the measurement unit.
Stoichiometry Test
We took a stoichiometry test and I'm pretty sure I failed I had no idea what I was doing I had forgotten everything by the time I got to 5th hour. It was a difficult test and I see that I need to study it more.
Metals Lab
It was definitely one of my favorite labs there were many unexpected reactions. These observations ranged from a milky bubbling to a dark brown, murky substance. The reactions that included calcium as a reactant tended to be very violent, and the majority of them produced smoke. Another reaction gave off a very disgusting odor. We couldn't tell which one as we filled the wellplate up, making it impossible to identify the exact reaction that was giving off that odor.


Decomposition
A decomposition reaction is when a more complex substance breaks down into its more simple parts. It is thus the opposite of a synthesis reaction. The way this is shown is, AB --> A+B
More Chemical Reactions
A chemical reaction is when two or more substances react together to create something else. Chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation.
| http://www.grandinetti.org/chemical-reactions |
Beginning Chemical Reactions
The substance initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants.
RedOx
We learned about the different types of RedOx. This includes synthesis, decomposition, single replacement reactions, and combustion. Redox reactions are reactions in which electrons have been transferred.
Tuesday, November 17, 2015
Empirical Formulas
The lesson about Empirical formulas was simple but the problems were difficult. Empirical formulas seem hard at first but are actually quite simple. It is just the molecular formula reduced to as small as it can go. You can also find the empirical formula using mass or percentage of an element.
 |
| https://www.chem.tamu.edu/class/majors/tutorialnotefiles/emp2c.gif |
Chloride Lab

It was just mass of the beaker, beaker plus zinc, and beaker plus acid and zinc. Pretty simple stuff.
Chemical Composition Test Reflection
This test was the worst test of my life. I have no doubt that I failed this test. My more intelligent friends were telling me it was hard so for me it was impossible. For most of the math questions I had no idea what I was doing. 10/10 Best test of all time. I hope I can save my grade.
| http://www.wallpaperfly.com/thumbnails/detail/20120628/meme%20sad%20crying%20white%20background%201920x1200%20wallpaper_www.wallmay.com_10.jpg |
Friday, November 13, 2015
Hydrated Compounds
The Hydrated compounds all have a water molecule in the formula. This contributes to the crystalline structure. Anhydrides must be hydrated first before they can become an anhydride.
_chloride.jpg) |
| https://upload.wikimedia.org/wikipedia/commons/4/4b/Cobalt(II)_chloride.jpg |
Molar Mass
In class we took about molar mass. Molar mass is the mass in grams of 1 mole of a substance. Basically converting the number of moles into mass in grams, or grams to moles.
| http://getchemistryhelp.com/wp-content/uploads/2013/05/ChemistryLessonMolarMass.jpg |
Chemical Composition Pre-test
This was by far the most impossible pre-test we have taken all year. I knew about 3 of the answers probably less actually. I had no idea what I was doing. I didn't know you could know the number of molecules in an element when given the mass the mass. This is probably going to be the lowest grade I have ever gotten.
Thursday, October 29, 2015
Moles and Stuff
We made moles in chemistry, yes that's right moles like the kind that dig holes in the ground. Apparently the number 6.02214179 × 10 23 in chemistry is a "mole" that's the scientific name. This was one strange project but I won't complain making a mole was fun.
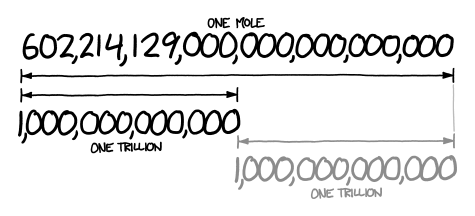 |
| https://what-if.xkcd.com/imgs/a/4/moles_number_length.png |
Measurement Quiz Reflection
I took the quiz and it was rather fast the quiz was short only 25 questions. I thought I did a good job it seemed very simple and straightforward I thought it was the easiest test of this year... Then I got my grade back and realized I had no idea what I was doing. The most confusing part for me was when a question had multiple operations such as multiplication then division and maybe a little addition or subtraction too. That confuses me very very much.
Significant Figures Lesson
In class we learned about significant figures and the importance of zeroes. I didn't know numbers could be more important than others but I guess I was wrong. I was able to do some in class practice which helped me understand it better, but it was still a little confusing at times. No matter what math or chemistry says I will always love zeroes.
| http://apchemcyhs.wikispaces.com/file/view/sigfig.jpg/85872249/560x336/sigfig.jpg |
Measurement pre-test
This was by far the hardest pre-test we have taken this year I knew maybe 3 of the questions and honestly I probably got them wrong. I had no idea how to figure out a lot of the questions and I am interested to learn about some of these new things such as "significant figures" I feel that this unit will be very mathematical and interesting.
| http://commonthreads.typepad.com/.a/6a00d83516641653ef017ee3fc6aa3970d-pi |
Aspirin Lab
We took a pre-test for the aspirin lab today but sadly ran out of time to complete it so we could not do the lab. The lab questions seemed much harder than the ones from Monday but I guess that's what happens when you wait too long.
| http://i.telegraph.co.uk/multimedia/archive/02945/aspirin_2945793b.jpg |
Monday, October 5, 2015
Final thoughts on Atomic Structure and Radioactivity
In this unit we learned about what happens to atoms over time. We learned about three different scientists who contributed to our knowledge of atoms, Dalton, JJ Thompson, and Ernest Rutherford. They all conducted different experiments that helped to understand how atoms work. We also discussed radioactive decay and how it affects the nucleus of the atom.
Tuesday, September 29, 2015
Half-Life and Forensic Archaeology Lab
Today we learned a new lesson about half-life. We had 567 squares and we flipped them 5 times until we could get them to "decay". This was honestly kind of annoying and took a long time with all of the counting and when you miss count it messes up the data. But it did help me to understand better how radioactive decay works.
Beanium Lab
I assumed by the name "Beanium" that it would have something to do beans. We used our newly learned skill of calculating mass to do this lab. Each bean was one isotope. We had to calculate the mass of each "isotope". This lab helped us learn and practice calculating average atomic mass.
Isotopes
Today we did a lab using beans to calculate atomic mass. Also we learned how to write an isotope in proper notation. I understand how to calculate the amount of subatomic particles. This was a new concept to me, but after taking notes and going over the lesson I understand this topic.
http://michiganbean.org/pinto-bean/
http://michiganbean.org/pinto-bean/
First things learned about Atomic Structure and Radioactivity
We first learned about different scientists and how they contributed to the atomic model. We learned how to calculate percentages. of certain elements. During the pre-test I was very confused but afterwards after the notes and lesson I understood the concepts.
Tuesday, September 15, 2015
First thoughts on Atomic Structure and Radiation
During the pretest I felt like I was back in kindergarten learning how to write letters for the first time, by that I mean I was clueless. This was a very difficult test for me. I have no idea what Beta or Alpha mean and I actually don't even know how any of this works.
Monday, September 14, 2015
Final thoughts about nomenclature
This project was hard to do very time consuming but simple to understand. Different plants have different active chemical ingredients and when used in the right way you can sort of "activate" those chemicals and it can help you in many ways and you can actually cure maladies like they used to before modern medicine.
| http://s560.photobucket.com/user/Chrystalanya/media/Textures/plant-leaves-texture.jpg.html |
What I learned about nomenclature
I learned about using plants with specific chemical ingredients to help cure certain injuries and sicknesses. It was very fun and cool to learn about how many uses plants can actually have. I very much enjoyed it and cannot wait for the next project.
Thursday, August 20, 2015
Introduction Page
Hi, my name is Tyler Stender and this is my Frontier Chemistry Project. I am a student at Francis Howell. I am a sophomore and it's just great. I enjoy doing homework and studying for my classes. Yeah not really but I have to because that's the way this system works.
 |
| http://gollumpus.blogspot.com/2014/09/movie-review-teenage-mutant-ninja.html |
Subscribe to:
Comments (Atom)
