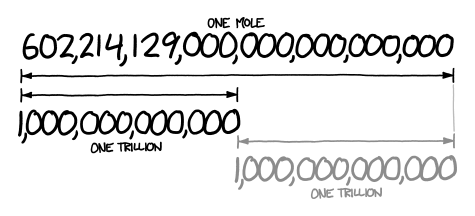 |
| https://what-if.xkcd.com/imgs/a/4/moles_number_length.png |
Thursday, October 29, 2015
Moles and Stuff
We made moles in chemistry, yes that's right moles like the kind that dig holes in the ground. Apparently the number 6.02214179 × 10 23 in chemistry is a "mole" that's the scientific name. This was one strange project but I won't complain making a mole was fun.
Measurement Quiz Reflection
I took the quiz and it was rather fast the quiz was short only 25 questions. I thought I did a good job it seemed very simple and straightforward I thought it was the easiest test of this year... Then I got my grade back and realized I had no idea what I was doing. The most confusing part for me was when a question had multiple operations such as multiplication then division and maybe a little addition or subtraction too. That confuses me very very much.
Significant Figures Lesson
In class we learned about significant figures and the importance of zeroes. I didn't know numbers could be more important than others but I guess I was wrong. I was able to do some in class practice which helped me understand it better, but it was still a little confusing at times. No matter what math or chemistry says I will always love zeroes.
| http://apchemcyhs.wikispaces.com/file/view/sigfig.jpg/85872249/560x336/sigfig.jpg |
Measurement pre-test
This was by far the hardest pre-test we have taken this year I knew maybe 3 of the questions and honestly I probably got them wrong. I had no idea how to figure out a lot of the questions and I am interested to learn about some of these new things such as "significant figures" I feel that this unit will be very mathematical and interesting.
| http://commonthreads.typepad.com/.a/6a00d83516641653ef017ee3fc6aa3970d-pi |
Aspirin Lab
We took a pre-test for the aspirin lab today but sadly ran out of time to complete it so we could not do the lab. The lab questions seemed much harder than the ones from Monday but I guess that's what happens when you wait too long.
| http://i.telegraph.co.uk/multimedia/archive/02945/aspirin_2945793b.jpg |
Monday, October 5, 2015
Final thoughts on Atomic Structure and Radioactivity
In this unit we learned about what happens to atoms over time. We learned about three different scientists who contributed to our knowledge of atoms, Dalton, JJ Thompson, and Ernest Rutherford. They all conducted different experiments that helped to understand how atoms work. We also discussed radioactive decay and how it affects the nucleus of the atom.
Subscribe to:
Comments (Atom)